It is known – Chemical Reactions Sustain Life! Some are favourable and can be facilitated without additional energy, while others need a source of energy to occur.
To help us explain, let’s imagine a dam; water accumulates upstream due to the dam’s presence. This water represents potential energy because once released, it can power a turbine that will produce electrical energy. This could represent a favourable chemical reaction, that is, water will go downstream whenever it can. But water does not go upstream, and if we need water to go upstream it needs to be pumped, we need energy to do that, in this we could draw a parallel with an unfavourable chemical reaction that requires energy to allow it to occur. In our world, the most common energy currency is electricity, which can be produced from various sources such as solar, hydroelectric, and fossil fuel combustion etc. Although life uses electricity to transmit information, it doesn’t use it as a universal energy source. All life forms on the planet use the same chemical energy currency, Adenosine triphosphate (ATP).

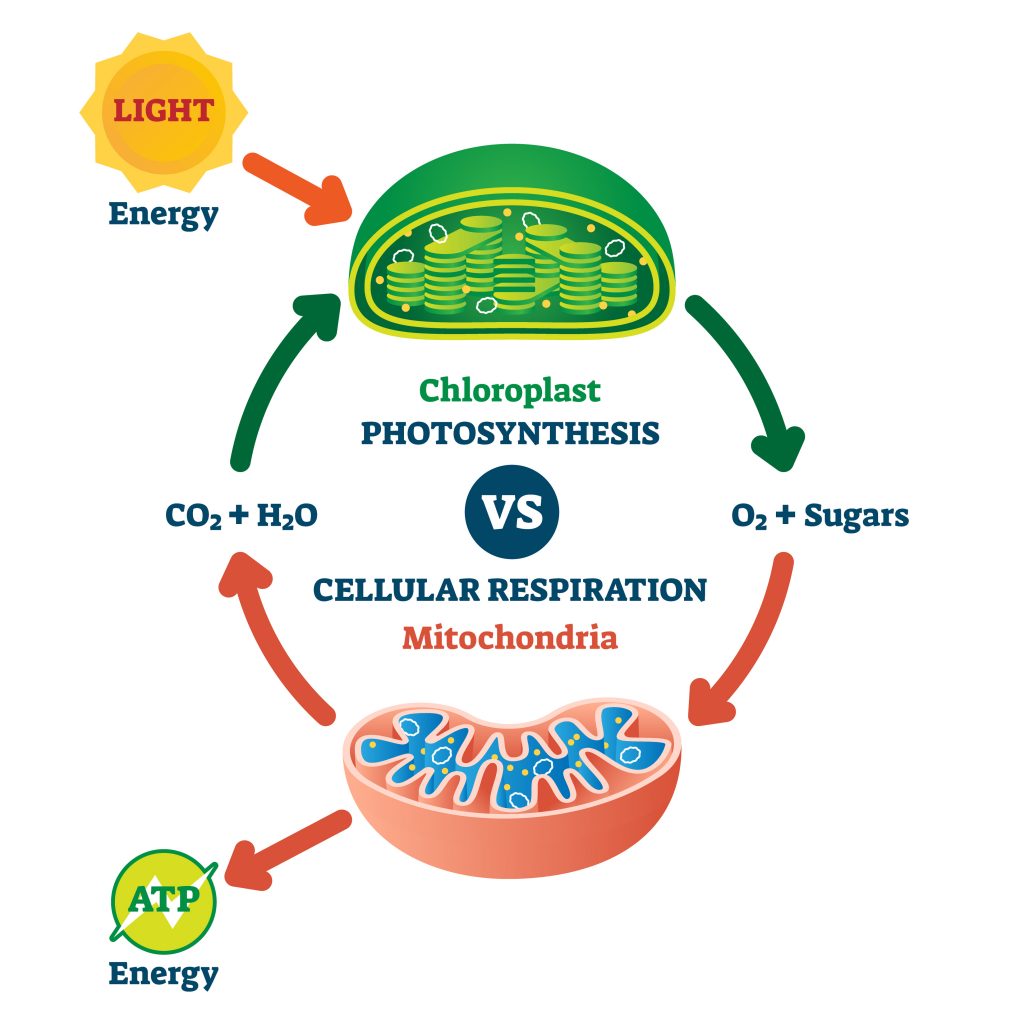

In keeping with the analogy of electricity production, ATP can be produced by various means, one of which is solar. ATP is produced in plants using solar energy in an organelle called chloroplast, while another cell organelle the mitochondria, provides the baseload ATP production in plants, animals, and fungi. Other processes in the cell can produce ATP and provide peak load power, the main ones being the phosphocreatine system for extremely fast supply of ATP in the very short term (much like a battery system), and glycolysis for a quick (if inefficient), production of ATP like a gas-fired power station. In this analogy the mitochondria are the nuclear central that provide the baseload and takes longer to adapt. In all cases, ATP is formed by adding a phosphate group to a molecule of Adenosine Diphosphate (ADP).
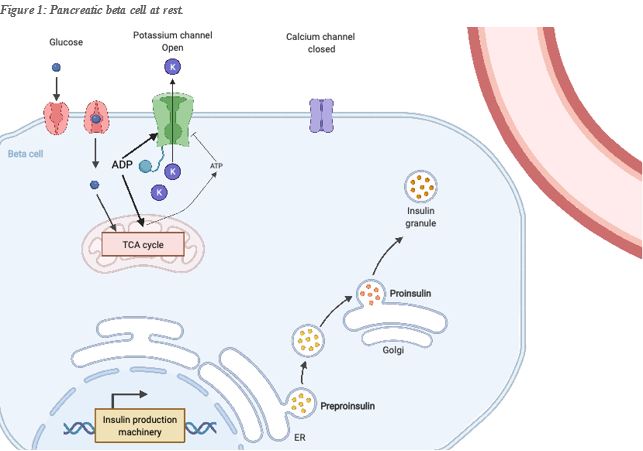
To illustrate the importance of ATP in cell physiology, let’s examine for example its function for insulin release. Insulin is produced by pancreatic Beta-cells and stored for future use in vesicles ready to be released in the bloodstream. Insulin promotes cellular glucose uptake, particularly in muscle and adipocytes (fat-storing cells), helping to maintain an optimum glucose level in the bloodstream. While insulin promotes glucose uptake, glucagon produced by the pancreatic alpha-cell promotes its release in the bloodstream. The Pancreatic Beta-cell secretes insulin into the bloodstream in response to glucose, a process that has everything to do with the relative level of mitochondrial ATP vs its precursor ADP in the cell. In the cellular membrane of the beta-cell, two channels are key to the secretion of insulin, one is the voltage-dependent Calcium channel which is closed at the normal beta-cell resting potential, and the second is an ATP sensitive potassium channel. This channel allows the exit of potassium from the beta cell, maintaining the proper resting potential. While ADP (the precursor of ATP), promotes its opening, ATP promotes its closing, and thus a change in membrane potential ad consequently the calcium channel’s opening. The entry of Calcium in the cell will trigger the fusion of the insulin vesicles with the cell membrane, and thus the release of insulin into the bloodstream.
So how is the level of glucose in the bloodstream sensed? As the glucose concentration in the blood increases (e.g. after a meal), the amount of glucose that enters the cell increases. Glucose is converted to pyruvate and is then transported to the mitochondria, which results in an increased ATP production by the mitochondria (Figure 2). The increased production of ATP translates into a rising concentration of ATP in the cell, coupled with a decrease in the concentration of ADP (ADP is consumed when producing ATP), which will close the ATP-sensitive potassium channel, inducing depolarisation of the cell membrane. This depolarisation enables the opening of the voltage-dependent calcium channel, enabling calcium to enter the cell. This in turn, causes the fusion of the insulin-containing vesicle with the cell membrane and the release of insulin into the bloodstream.
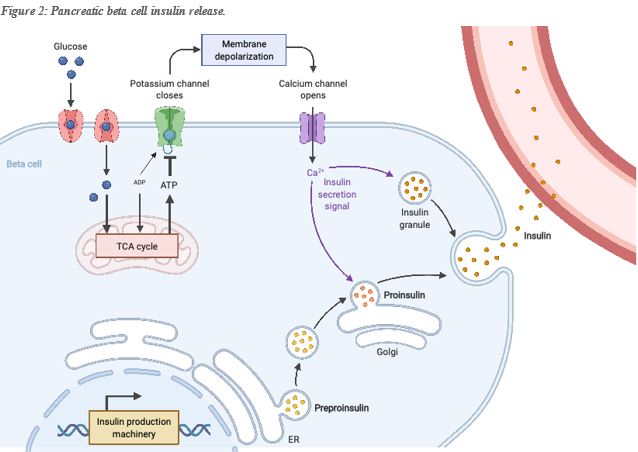
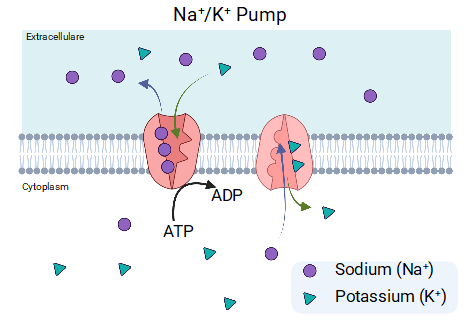
In the example above (figure 1), potassium exits the cell because there is always more potassium inside the cell than outside the cell, however in the case of calcium, the calcium concentration in the cytoplasm is maintained low compared with the outside concentration, thus the opening of calcium channel will always trigger an entry of calcium into the cell. Once again, ATP is necessary to maintain the normal relative potassium, sodium, and calcium concentration inside and outside of the cell. In pretty much all cell type’s membranes, there exists a sodium-potassium pump powered by ATP. Three sodium ions are exported for every ATP molecule that the pump uses, and two potassium ions are imported into the cell. These pumps ensure that the extracellular concentration of sodium is always higher outside the cell, whereas the concentration of potassium is always higher inside the cell. Calcium is a critical modulator of enzymatic activities, thus its concentration inside the cell is highly regulated, especially in the cell cytoplasm. Therefore, an ATP dependent calcium pump is found in the cell membrane and will pump one calcium outside the cell for every ATP it consumes. The regulation of Calcium concentration in the cell is essential, and thus, additional mechanisms exist for its control, however, they are still reliant on there being enough ATP available to facilitate it.
The availability of ATP for protein folding is another example, as it is not only the protein amino-acid sequence that determines properties or activity but also its 3D structure that defines it. In any organism, each gene codes for one or more proteins, and to build proteins, each cell needs to transcript DNA to RNA, and then read (translate) the RNA to build the protein sequences. The young protein then needs to be properly folded to acquire proper function. Protein folding is a very complex process, but it is dependent on ATP, and if there is not enough ATP present, the protein is likely to be misfolded. The misfolding of protein can affect the recognition of self by the immune system as well as cellular function itself. However, terminally misfolded proteins are generally directed to recycling by protease before they leave the reticulum endoplasmic and thus, mechanisms exist within the cell to avoid undesirable consequences even though they are not fool proof.
ATP as an energy source also enables muscle contraction and relaxation, it enables a heart to beat, and the reabsorption of important metabolites by a kidney. As a signalling molecule, it is used as a neurotransmitter by some neurons, but it is also one of the building blocks of RNA, and the precursor of dATP (deoxyadenosine triphosphate), a building block of DNA. Although we have tried to use familiar examples to highlight the importance and relevance of ATP in biology, it is species indifferent and virtually all life works the same way. The primary function of mitochondria is to convert chemical energy into a biological energy, to be used by every cell throughout the entire body, regardless of species. The ability to produce more ATP or less, is dependent on a great many things however, it is directly related to the efficient functioning of the mitochondria.
Hydrogen is a major constituent of any life form and represents more than three of every five atoms in animal species, and just under half of all the atoms in plants. Hydrogen, both its protons and electrons, appears to not only enable, but also optimise energy production by mitochondria and chloroplast. The significance of hydrogen in biology cannot be underestimated as it is part of the very first step in mitochondrial function as well as the last, be it part of a proton pump, the transfer of electrons, and the completion of redox balance and homeostasis.
There are an estimated 37.2 trillion cells in the body and each cell contains between 2 and 2500 mitochondria each possessing 17,000 ATP assembly line. Thus, it is estimated that there are about 10 million billion (10,000 trillion) mitochondria in an adult human for example! The oxidative phosphorylation of one molecule of glucose consumed by the cell results in the production of 32 ATP by the mitochondrion.
Molecular hydrogen supplementation has the ability to increase mitochondrial ATP production by more than 50% while decreasing the production of superoxide by the first respiratory complex, thus increasing cell energy availability while decreasing harmful reactive oxygen species. The ability to increase the production of ATP while reducing the production of reactive oxygen species and reducing the need to repair the damage they cause, represents an advancement in biological strength and health that could not be more relevant in today’s world.
Very recently it was demonstrated that H2 supplementation suppressed superoxide production1 by complex I, its main producer. Furthermore, Ishihara et al. suggested that H2 donated electrons directly in the Q chamber of complex I1, consequently making 2 more H+ available also. Two main mechanisms are possible, but given that Hydrogen evolution (the production of H2 from two protons) by complex I in plants have recently been demonstrated2, the most likely mechanism is that complex I acts as an oxygen insensitive hydrogenase capable of both using Hydrogen to reduce ubiquinone to ubiquinol, and to accept electrons from ubiquinol and evolve Hydrogen gas from two protons. Regardless of how H2 participates in the respiratory chain, it is demonstrated that H2 supplementation translates into more than a 50% per min increase in ATP production by the mitochondria3, as well as the significant reduction of superoxide at the same site. It is also known that H2 supplementation upregulates superoxide dismutase and catalase. This taken together with the significant reduction of superoxide production in the matrix by complex I, represents enormous biological benefit to the mitochondria. Furthermore, the ATP increase appears to be partially a least, to be uncoupled from nutrient intake. An increase in ATP production by the mitochondria following H2 supplementation, means that cells can divert the nutrients not used to produce energy, into the production of the building blocks of the cells. This further explains why crops supplemented with hydrogen can invest more energy into growth and production as well as the increased rate of wound repair and recovery from exhaustion and injury in animal species.
Peer-reviewed scientific documents now run into the thousands. Taken collectively they clearly identify that the deficiency of hydrogen, and consequently ATP, in biological organisms costs the world well over 100 trillion dollars in unnecessary expenses or lost income every single year. It underpins the health potential and lifespan of all things, ultimately and consequentially our entire biosphere (including its climate) as we know it. From a scientific viewpoint, it is only now that we are beginning to realise the importance of molecular hydrogen in biology and its tremendous benefit. From little things, big things come, and it is from the smallest atom that comes a planet of life.
References:
1. Ishihara, G., Kawamoto, K., Komori, N. & Ishibashi, T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem Biophys Res Commun 522, 965-970 (2020).
2. Xin Zhang et al. Mitochondria in higher plants possess H2 evolving activity which is closely related to complex I.
3. Gvozdjáková, A. et al. A new insight into the molecular hydrogen effect on coenzyme Q and mitochondrial function of rats. Canadian Journal of Physiology and Pharmacology 98, 29-34 (2020).





