David Guez – PhD Neurobiology – Ecotoxicology
Jim Wilson – Director – Founder
Adenosine triphosphate (ATP) is often described as the energy currency of life, and every cell needs to produce it to sustain its normal function. ATP is mainly formed into two cell compartments the cytoplasm periphery adjacent to the cell membrane and the mitochondria generally situated closer to the nucleus and the reticulum endoplasmic. While peripherical ATP production uses aerobic glycolysis and provides a fast response to rapid change in ATP demand, the mitochondria provide a large amount of ATP and is less sensitive to quick changes of demand. They can be described as satisfying the base-load demand1. Interestingly manipulation of peripherical ATP demand, for example, by inhibition of Na+/K+ membrane pump, translates into a decrease in glycolysis, while manipulation macromolecule synthesis translates into changes in respiration rate and thus changes in ATP by the mitochondria1.



The Respiration Chain:
The mitochondrion is a cell organelle enclosed by a double membrane – an outer membrane and an inner membrane – separated by the intermembrane space. It is on the inner membrane surrounding the DNA containing matrix that are localised the respiratory complexes responsible for ATP production. The respiratory chain comprises five protein complexes, Complex I, II, III, IV and V (Figure 1). The general purpose of the respiratory chain is to pump protons (H+) from the matrix to the intermembrane space, resulting in a larger concentration of H+ (Hydrogen ions) in the intermembrane space than in the matrix, so in creating a chemical and an electrical gradient. The creation of this gradient will provide the energy necessary for the synthesis of ATP by the ATP synthetase (complex V) following the terminal electron acceptor role of Oxygen in complex IV. The re-entry in the matrix of 3-4 moles of H+ at complex V, converts into the production of one mole of ATP. The motrice force necessary to pump protons across the membrane is provided by electron transfer (figure 1).
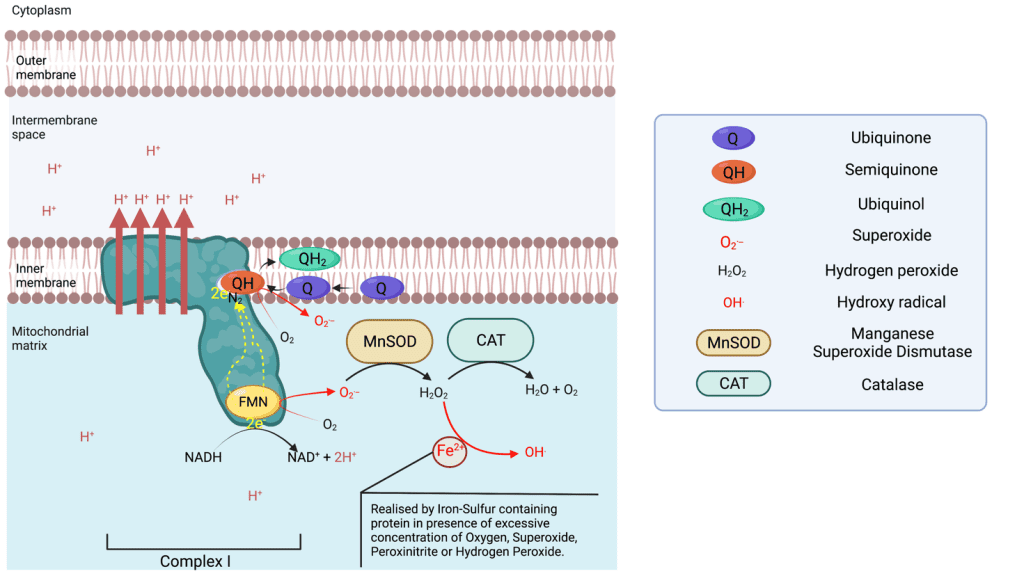
Complex I:
Complex I is an NADH : ubiquinone oxidoreductase, and is one of the largest membrane-bound enzymes in the cell. Complex I contains 44 subunits in mammals2, including 14 core subunits enabling its catalytic activities and conserved from bacteria to humans3,4. Complex I catalyse NADH oxidation to NAD+ on the matrix side, capturing 2 electrons that are going to be transferred toward the membrane domain along a Flavin Mononucleotide (FMN) located near the site of NADH oxidation and a chain of iron-sulfur clusters up to the N2 cluster from which the electrons are going to be donated to the Ubiquinone-10 (Q10 or Q) in the Q chamber, thus reducing the lipophilic Ubiquinone to Ubiquinol (QH2). QH2 will transport the electron across the inner membrane and donate them to complex III. The reduction of Ubiquinone to ubiquinol provides the energy necessary to transport four protons (Hydrogen ions) across the complex I membrane domain5. It is of note that NAD+ is reactivated to NADH via Beta oxidation of fatty acids and the tricarboxylic acid cycle taking place in the mitochondrial matrix.
Complex I is able to leak electrons at two sites 6 and transfer them to Oxygen forming superoxide ions (O.-) during the transfer of electrons from the NADH oxidase to the N2 cluster. The superoxide produced is found in the matrix, which can be converted in the matrix to hydrogen peroxide via a manganese superoxide dismutase (MnSOD). Complex I is the main source of ROS (reactive oxygen species) in the respiratory chain. If the production of superoxide is not balanced out by the activity of MnSOD, superoxide can deactivate complex I7. Superoxide is also the primary source of the very destructive Hydroxyl radical.
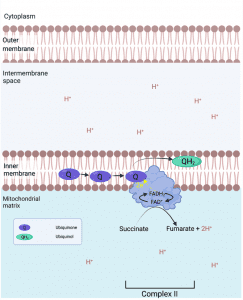
Complex II:
Complex II is a Succinate : ubiquinone oxidoreductase, and is composed of 4 subunits. The two largest subunits function as succinate dehydrogenase, oxidising succinate to fumarate. The two electrons freed are transferred to a covalently bound FAD (Flavin adenine dinucleotide)8 reducing it to FADH2, from there the electrons are transferred to be ultimately donated to ubiquinone Q reducing it to ubiquinol. Complex II is not a major leakage site but may produce some superoxide on the matrix side of the intermembrane. Complexe II does not pump H+.
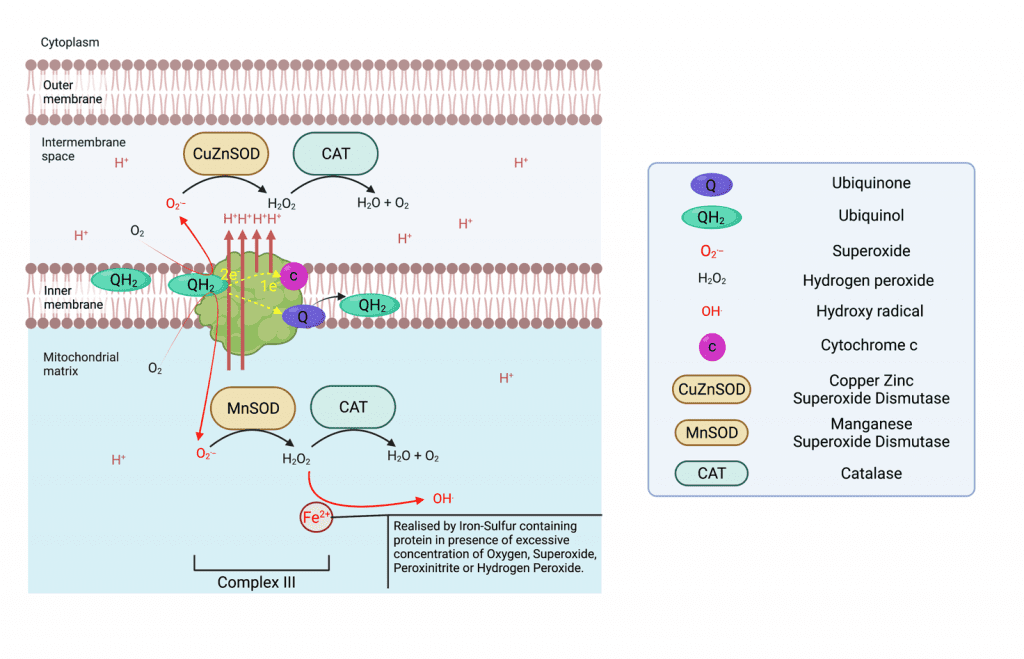
Complex III:
Complex III is a Ubiquinol cytochrome c reductase, and is a symmetrical dimer that contains 11 subunits each in mammals. Complex III receive successively two ubiquinol in the Q0 chamber each time, transferring one electron to cytochrome c that will transfer them again to complex IV, and one electron to its Qi site where ubiquinone is reduced to ubiquinol in a process called the Q cycle. So, one Q cycle sees the oxidation of two ubiquinol to ubiquinone at site Q0, the reduction of cytochrome c and the reduction of one ubiquinone to ubiquinol Qi. The transfer of electrons provides the energy necessary to transfert 4 H+ (4 x Hydrogen ions) to the intermembrane space (two of which are pumped from the matrix). Complex III can produce superoxide at each Q site resulting in superoxide release both in the intermembrane space where a copper-zinc superoxide dismutase (CuZnSOD), converts it to hydrogen peroxide. At the same time, the MnSOD in the matrix serves the same purpose6.
Complex IV:
Complex IV is a Cytochrome c oxidase, and is composed in mammals of 13 subunits. It transfers electrons from cytochrome c to the terminal electron acceptor O2 (Oxygen gas) to generate H2O, pumping four H+ (4 x Hydrogen ions) from the matrix to the intermembrane space. It is in this complex that Oxygen plays its role in the creation of ATP and mitochondrial function as the terminal electron acceptor at the end of the electron transport chain following the pumping of 12 hydrogen ions.
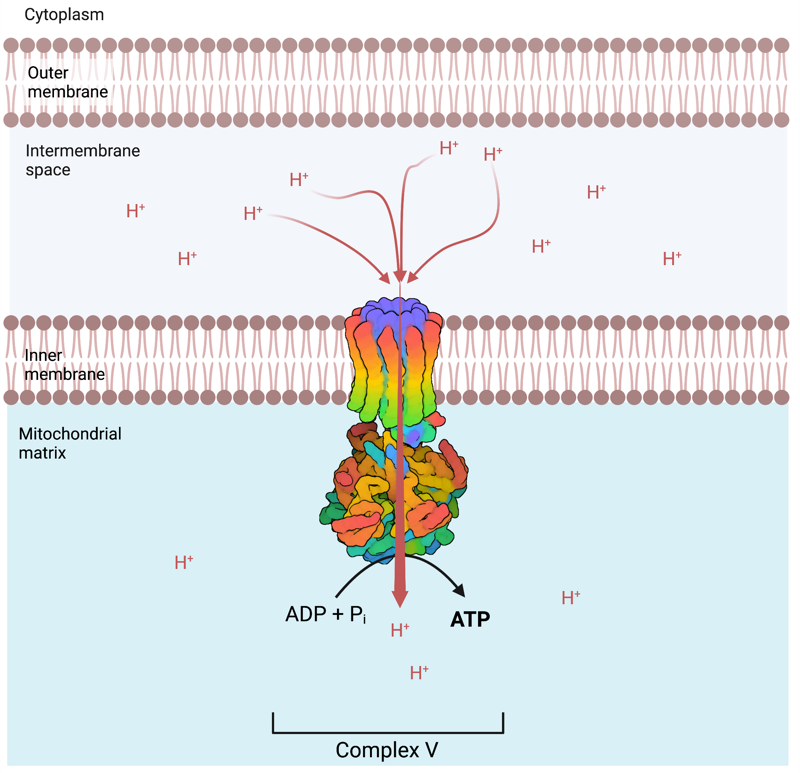
The ATP Synthase:
The ATP synthase sometimes called Complex V, is a mitochondrial enzyme localized in the inner membrane adjacent to the respiratory complexes. It catalyzes the synthesis of ATP from ADP and phosphate, using the energy derived from the re-entry flux of protons (H+) in the matrix from the intermembrane space. Thus, the respiratory chain enabled accumulation of H+ (Hydrogen ions) in the intermembrane space is the power that enables ATP synthesis in the mitochondria. The energy needed to pump H+ from the mitochondrial matrix to the intermembrane space is naturally derived from the metabolism of nutrients that we consume, such as the tricarboxylic acid cycle (also called the Kreb cycle), and the Beta oxidation of fatty acids or glutaminolysis. It is therefore directly associated with both the food and toxins ingested on a daily basis, that will determine the availability of the required cellular and mitochondrial ingredients, that enables an increase or decrease of ATP production.

Ultimately, the more H+ that are pumped into the intermembrane space, and the greater the difference with the H+ concentration in the matrix, the more ATP that can be produced by the ATP synthase and in consequence, the more ATP that is available to sustain cellular processes, and of course, life itself. Generally speaking, the less ATP produced, the less resilient an organism can be.
The Action of Molecular Hydrogen (H2) Supplementation on the Respiratory Chain:
Very recently it was demonstrated that H2 supplementation suppressed superoxide production9 by complex I, its main producer. Furthermore, Ishihara et al. suggested that H2 donated electrons directly in the Q chamber of complex I9, consequently making 2 x H+ available also. Two main mechanisms are possible, but given that Hydrogen evolution (the production of H2 from two protons) by complex I in plants have recently been discovered10, the most likely mechanism is that complex I acts as an oxygen insensitive hydrogenase capable of both using Hydrogen to reduce ubiquinone to ubiquinol, and to accept electrons from ubiquinol and evolve Hydrogen gas from two protons.
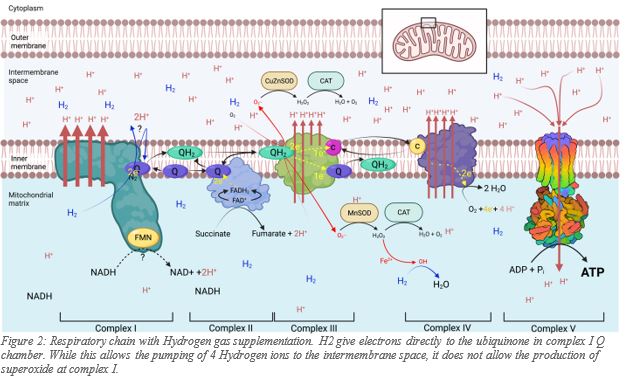
Regardless of how H2 participates in the respiratory chain, it is demonstrated that H2 supplementation translates into more than a 50% per min increase in ATP production by the mitochondria11, as well as the significant reduction of superoxide at the same site. The significant reduction of superoxide production in the matrix by complex I, represents enormous biological benefit to the mitochondria. Furthermore, the ATP increase appears partially a least, to be uncoupled from nutrient intake. An increase in ATP production by the mitochondria following H2 supplementation, means that cells can divert the nutrients not used to produce energy, into the production of the building blocks of the cells.
Refences:
1. Epstein, T., Xu, L., Gillies, R. J. & Gatenby, R. A. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab 2, 7 (2014).
2. Rodenburg, R. J. Mitochondrial complex I-linked disease. Biochim Biophys Acta 1857, 938-945 (2016).
3. Bridges, H. R. et al. Structure of inhibitor-bound mammalian complex I. Nat Commun 11, 5261 (2020).
4. Zhu, J., Vinothkumar, K. R. & Hirst, J. Structure of mammalian respiratory complex I. Nature 536, 354-358 (2016).
5. Zhang, X. C. & Li, B. Towards understanding the mechanisms of proton pumps in Complex-I of the respiratory chain. Biophysics Reports 5, 219-234 (2019).
6. Zhao, R. Z., Jiang, S., Zhang, L. & Yu, Z. B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 44, 3-15 (2019).
7. Indo, H. P. et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J Clin Biochem Nutr 56, 1-7 (2015).
8. Dourado, D. F. A. R., Swart, M. & Carvalho, A. T. P. Why the Flavin Adenine Dinucleotide (FAD) Cofactor Needs To Be Covalently Linked to Complex II of the Electron-Transport Chain for the Conversion of FADH2 into FAD. Chemistry 24, 5246-5252 (2018).
9. Ishihara, G., Kawamoto, K., Komori, N. & Ishibashi, T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem Biophys Res Commun 522, 965-970 (2020).
10. Zhang, X. et al. Hydrogen evolution and absorption phenomena in plasma membrane of higher plants. (2020).
11. Gvozdjáková, A. et al. A new insight into the molecular hydrogen effect on coenzyme Q and mitochondrial function of rats. Canadian Journal of Physiology and Pharmacology 98, 29-34 (2020).